Written by: JI Xuan
Translated by: DU Yufei
Edited by: William Mosteller
Date: 08-29
HIT News (Ji Xuan/news Liu Yang/photo) On August 28th, Professor HUANG Zhiwei, from HIT’s School of Life Sciences, together with his team, published an article entitled "Structural basis of assembly of the human TCR-CD3 complex" in the online journal, Nature. In this study, the high-resolution cryoelectron microscopic structure of the human TCR-CD3 complex (containing all of the 8 subunits) was first analyzed. Through structural analysis, the molecular mechanism of recognizing and assembling the TCR-CD3 complex in the outer and inner membranes was revealed, which answers the basic scientific questions about the structure of T cell receptors in the field of immunology. It is of great scientific significance to analyze the molecular mechanism of T cell activation, and also provides the key structural basis for the development of immunotherapy based on T cell receptors.
Professor ZHANG Xue, president of Harbin Medical University, spoke highly of the research results of Professor HUANG, believing that it is the first time to reveal the structure of the T cell receptor complex in the world and a major original discovery made by Chinese scientists in the basic research of immunology.
T cells are the key cells of the adaptive immune system in vertebrates and play a key role in viral infection, cancer, and autoimmune diseases. The process of T-cell immune response is as follows: TCR first recognizes the antigen-peptide-bound MHC complex (pMHC) on APCs, then it transmits the antigen signal to the intracellular ITAM region of the ζsubunit of CD3 through its binding co-receptor (CD3), thereby initiating an intracellular cascade of T-cell immune signaling pathways to kill pathogenic infected cells or tumor cells.
TCR of most mature T cells (about 95%) consists of two heterodimeric peptides linked by disulfide bonds. The variable regions (Vα and Vβ) of the TCRα/β are responsible for the recognition of antigen signals, and TCRα/β forms a TCR-CD3 receptor complex with CD3, a co-receptor signaling with 6 subunits, including γ/ε, δ/ε’andζ/ζ’. The complex determines the development, activation, and immune response of T cells to pathogens. In the past two decades, how the TCR extracellular variable region recognizes various antigens has been deeply studied, but the structural basis of the TCR-CD3 complex assembly and signal transduction, as one of the basic scientific problems of cellular immunity, is still unknown.
The team first screened different human cell banks for target TCRs, purified protein complexes by chemical cross-linking, and analyzed the high-resolution structure of the first human-derived TCRα/β-CD3 complex (Figure 1) by freeze-electron microscopy. The structure of the TCR-CD3 complex contains a complete extracellular domain (ECD) and all transmembrane regions. The structure of the complex shows that it is composed of the octameric subunits of TCRα/β:CD3γ/ε:CD3δ/ε’:CD3ζ/ζ’ by the proportion of 1:1:1:1, which is consistent with previous biochemical studies. The extracellular region of the TCR-CD3 complex is composed of the constant region of TCRα/β and the combination of the ligating peptides that link extracellular and intracellular membranes and the two dimer modules of the γ/ε和δ/ε’ of CD3. The assembly of the extracellular region of TCR-CD3 complex forms a cubic symmetric structure near the outer side of the cell membrane, and the constant region of the TCRβ subunit is located at the center of the cubic symmetric structure. The inner part of CD3 membrane is composed of the two transmembrane helices of the ζ/ζ’subunit and the transmembrane helices of the γ/ε and δ/ε’subunits, which combine to form a barrel-like structure. Transmembrane assembly of the TCR-CD3 complex is formed by the insertion of two transmembrane helices of the TCRα/β into the CD3 barrel-like structure by hydrophobic and ionic interaction (Figure 2). Therefore, the binding peptides of each subunit of TCR-CD3 near the membrane and the strong interaction of the intramembrane region play a key role in the assembly of the whole complex. The team compared the structure of the complex with that of the TCRα/β extracellular domain bound to pMHC and found that the binding of pMHC did not cause significant changes in the TCRα/β structure.
"This work represents an important milestone in the study of the molecular mechanism of cellular adaptive immunity. By elucidating the structure of the first T cell receptor assembled on the membrane and its CD3 co-receptor, we have greatly enhanced our understanding of the activation mechanism of T cell antigen recognition responses," the reviewers said.
Professor SHI Yigong, president of West Lake University and an academician of the Chinese Academy of Sciences, also spoke highly of the study. SHI believed that "the mystery of the structure of the T cell receptor complex has always been an important scientific problem that the world's top scientists dream of solving in cell adaptive immunology, and the analysis of the complex structure by HUANG Zhiwei's team from HIT is an important milestone in understanding the mechanism of cell adaptive immunity".
Doctor DONG De and Master LIN Jianquan from HIT and Doctor ZHENG Luqin from Peking University are the first authors of the article. Professor HUANG Zhiwei and Professor GAO Ning from Peking University are co-corresponding authors of the paper. Technician ZHANG Bailing and postdoctoral researcher ZHU Yuwei of the College of Life Sciences of HIT participated in this research. This program is supported by the National Natural Science Foundation of China and the Young Scientist Studio of Harbin Institute of Technology.
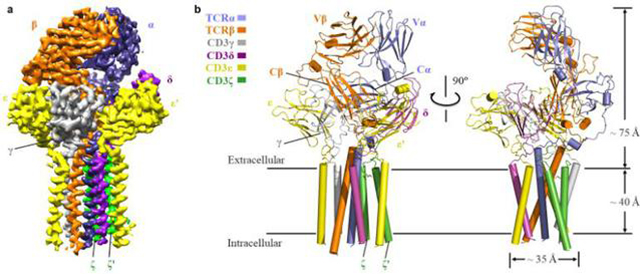
Figure 1. The whole structure of the TCR-CD3 complex
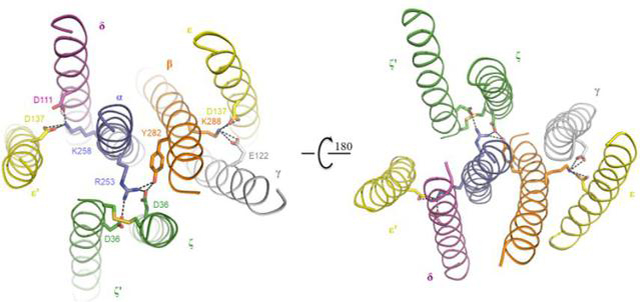
Figure 2. A detailed view of the intracellular assembly of the TCR-CD3 complex

Professor HUANG Zhiwei and his team members

Professor HUANG introducing the study.

Professor HUANG taking an interview.


