Professor Yongsheng Yu from the School of Chemical Engineering and Technology, a member of the National Key Laboratory of Urban Water Resources and Water Environment, has collaborated with Professor Shaojun Guo’s team from Peking University to achieve significant progress in the field of high-entropy alloy energy electrocatalysis. Their research, titled Single-Atom Mo-Tailored High-Entropy-Alloy Ultrathin Nanosheets with Intrinsic Tensile Strain Enhance Electrocatalysis, has been published in Nature Communications. This achievement enhances the electrocatalytic efficiency of methanol oxidation by altering the performance of high-entropy alloy electrocatalysts through single-atom doping and strain engineering.
Direct methanol fuel cells (DMFCs) are recognized as ideal power sources for portable electronic devices and electric vehicles due to their high energy conversion efficiency, environmental friendliness, and convenient transport. Platinum-based catalysts are commonly used for anodic methanol oxidation reaction (MOR); however, the high dosages of Pt-based catalysts and poor tolerance to CO adsorbates (COads) (the notorious intermediate blamed for poisoning Pt active sites in MOR) severely hinder the large-scale commercialization of DMFCs. To improve catalyst efficiency, it would be beneficial to further promote the oxidation of CO intermediates or switch the reaction to CO-free pathway.
Research has shown that high-entropy alloys (HEAs) are promising materials for the freedom from CO poisoning and improving MOR performance because of their robust capacity for isolating Pt atoms and their expansive and modulable compositional space. Despite significant advancements in the design of high-entropy alloys in recent years, precisely designing and optimizing HEAs at the atomic level to improve MOR activity, CO tolerance, and stability remains a substantial challenge.
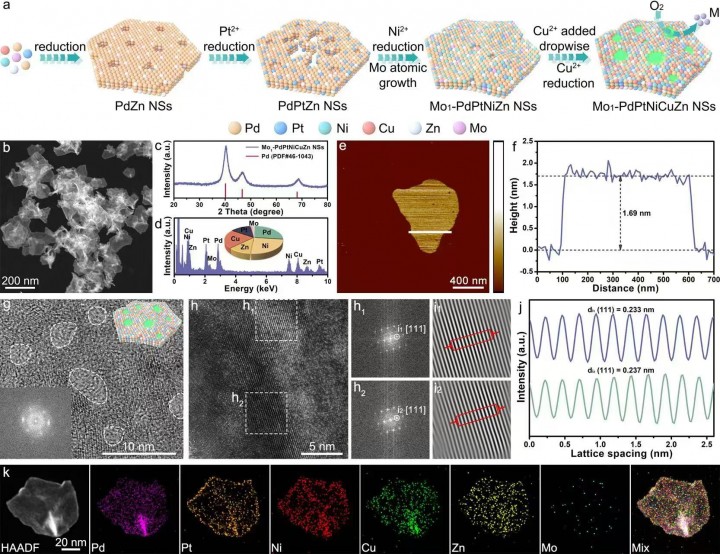
In response to this, the team synthesized oxophilic molybdenum single-atom modified platinum-based high-entropy alloy ultrathin nanosheets, significantly enhancing the electrooxidation of MOR. The representative Mo1-PdPtNiCuZn single-atom high-entropy alloy nanosheet catalyst exhibited excellent mass activity (24.55 A per milligram of platinum and 11.62 A per milligram of platinum plus palladium) and long-term stability. The team investigated the mechanism behind the enhanced MOR performance of the Mo1-PdPtNiCuZn single-atom high-entropy alloy nanosheet catalyst through in-situ spectroscopy and theoretical calculations. The results indicated that the oxophilic molybdenum single atoms and tensile strain further adjusted the electronic structure of the isolated platinum active sites in the high-entropy alloy hosts, optimizing the adsorption behavior of key reaction intermediates, thereby enhancing formate-dominated MOR electrocatalysis. This research establishes a new paradigm for single-atom-modified high-entropy alloys, advancing the design of atomically precise catalytic sites and paving the way for the development of CO-tolerant fuel cell electrocatalysts.

Paper Link: https://www.nature.com/articles/s41467-024-45874-z


