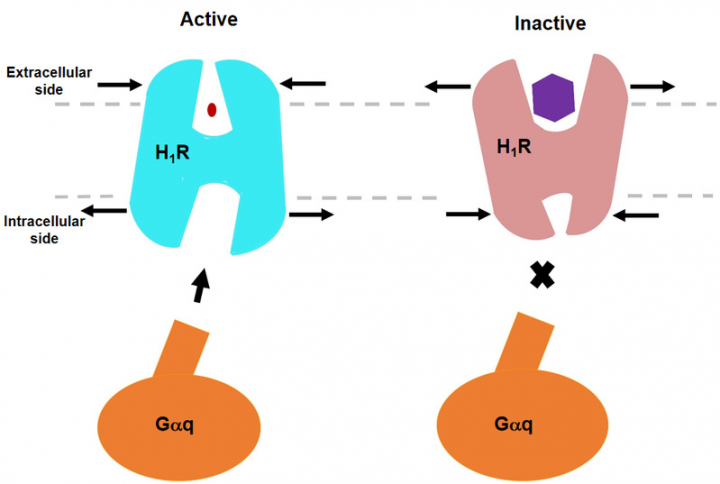
HIT News— On April 7, He Yuanzheng's research team from HIT Center for Life Science published a paper in Nature Communications, revealing Cryo-EM structure of the human histamine H1 receptor/Gq complex for the first time. Histamine is a biogenic amine, which exerts a variety of physiopathological effects by activating four known histamine receptors. Histamine receptors are divided into four types: H1R, H2R, H3R and H4R, and they belong to the class A G-protein-coupled receptor (GPCR) superfamily. Studies have shown that H1R and H2R are targets for the treatment of allergies and gastric acid-related diseases, while H3R and H4R have a great clinical potential for dementia, asthma, inflammatory bowel disease, and rheumatoid arthritis. The receptor bind to histamine to recruit heterotrimeric G-protein and trigger a downstream signaling cascade. H1R is mainly coupled with Gq protein to activate phospholipase C, increasing inositol phosphate and intracellular calcium levels; H2R is coupled with Gs protein; H3R and H4R stimulate cAMP for signal transduction through Gi/o protein.
Histamine is the key mediator of immune allergy (type I allergy) and the major trigger of H1R-mediated allergic diseases. Antihistamine drugs have been developed for more than half a century, and they have always been the first choice for the treatment of allergic diseases. The first-generation antihistamines have high blood-brain barrier permeability and low receptor selectivity, which can cause drowsiness, dry mouth and other side effects. The second- and third-generation antihistamine can significantly reduce brain permeability, but still have shortcomings, such as low affinity to the receptor and cardiotoxicity. At present, the most successful antihistamine drug design is a macromolecule with a basic amino group, which is very different from the imidazole ring and ethylamine side chain of histamine, but the mechanism by which those macromolecular antihistamines block the H1R signal still remains unclear.
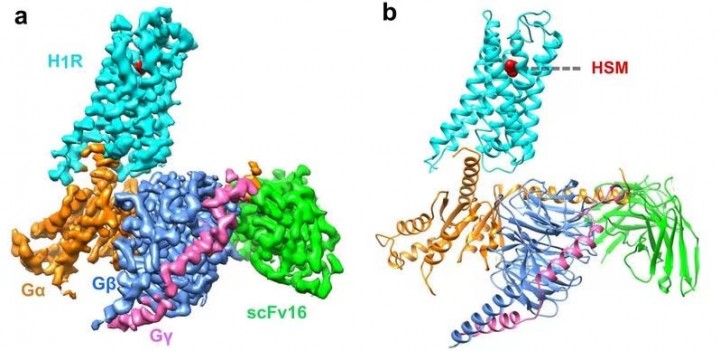
An early study has revealed the inactive structure of H1R binding to the first-generation of antihistamine peptides. However, the exact mechanism by which the ligand induces receptor activation is still unclear. This paper demonstrates the complex structure of human H1R and Gq protein by cryo-electron microscopy (cryo-EM). The structure indicates that histamine activates the receptor by interacting with the key residues of transmembrane domain 3 (TM3) and TM6, squashing the binding pocket on the extracellular side, opening the cavity on the intracellular side, and recruiting Gq protein. The key residue Y4316.51 of TM6 is mutated to a positively charged residue R or K to make it interact with the key negatively charged D1073.32 of TM3, which mimics the effect of histamine in pulling TM6 toward TM3 in the binding pocket, resulting in the higher basic activity of the receptor. On the contrary, it was found that antihistamines (inverse agonists) use their large groups to push TM6 and TM3 apart, expanding the ligand-binding pocket and creating a model of “squeezing to activate and expanding to deactivate”. Comparison with other receptor/G-protein complexes reveals features of Gq coupling, including the interaction between intracellular loop 2 (ICL2) and the αN-β junction of Gq/11 protein, and the involvement of TM7-helix 8 (H8) bound to G protein. In addition, a dramatic outward translational movement of the αN of the Gq protein is observed upon receptor engagement. These findings could help to understand the G coupling selectivity and provide clues for designing novel antihistamines. This is also the first time to present the structure of the Gq complex.
Xia Ruixue, an experimental technician in He Yuanzheng's research group, and Wang Na and Xu Zhenmei, Ph. D. students, are the co-first authors. Student Song Jing participated in the research work. Zhang Anqi and Guo Changyou, HIT cryo-EM Platform engineers, jointly participated in the collection of the structural data, and some of the data collection was done with the assistance of the Cryo-EM Center of the Shuimu Bioscience (Beijing). Professor He Yuanzheng is the corresponding author of this paper.
Original link of the paper :
https://www.nature.com/articles/s41467-021-22427-2#MOESM1