Professor Kuikun Yang’s group at the School of Life Science and Technology of Harbin Institute of Technology has made significant progress in the field of inflammation suppression in ischemic stroke. Their findings titled Neutrophil Membrane-Camouflaged Polyprodrug Nanomedicine for Inflammation Suppression in Ischemic Stroke Therapy were published in Advanced Materials, providing new insights into the application of nanomedicine in ischemic stroke treatment.
Ischemic stroke is characterized by brain tissue damage due to the sudden blockage of blood vessels in the brain, resulting in high incidence, mortality, disability, and recurrence rates. Current treatments for ischemic stroke focus on early reperfusion to rescue damaged neurons at the stroke site. However, reperfusion can lead to inflammatory responses with local elevation of Reactive Oxygen Species (ROS), thus resulting in secondary neural tissue damage. Therefore, effective control of brain ischemia-reperfusion injury is crucial for protecting brain tissues at the stroke site and restoring their function.
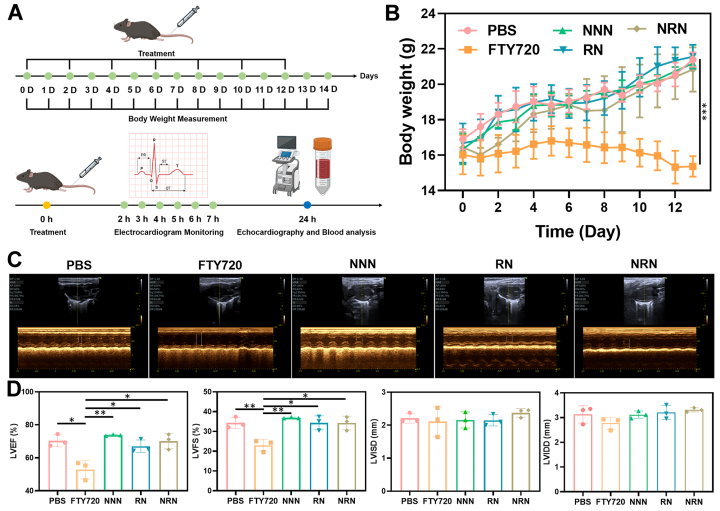
Fingolimod, an FDA-approved Sphingosine-1-Phosphate Receptor (S1PR) modulator for the clinical treatment of multiple sclerosis, has shown potential to alleviate stroke-induced neurological damage. However, the protective effects of fingolimod on neural damage are dose-dependent, while the presence of the blood-brain barrier limits the entry of most fingolimod into the stroke site to exert its effects. Additionally, high doses of orally or intravenously administered fingolimod can lead to severe cardiac toxicity, significantly restricting its application in clinical stroke treatments.
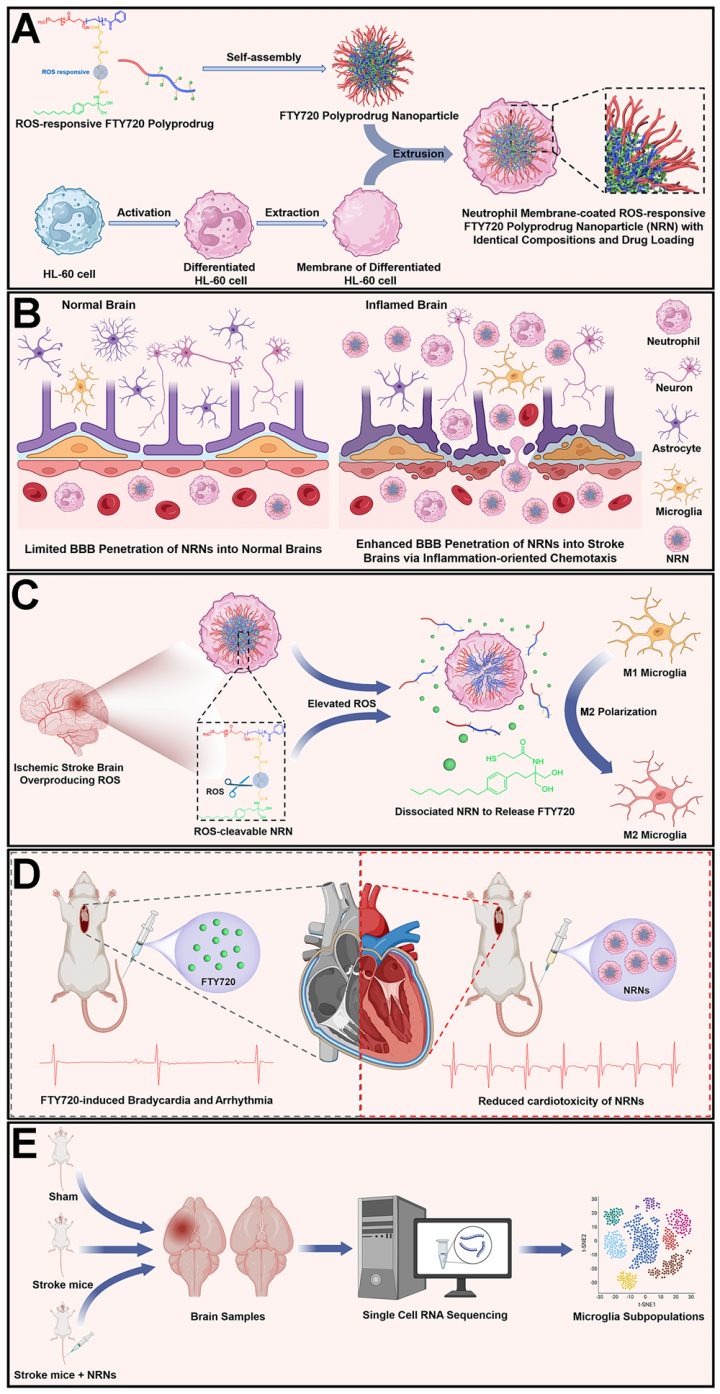
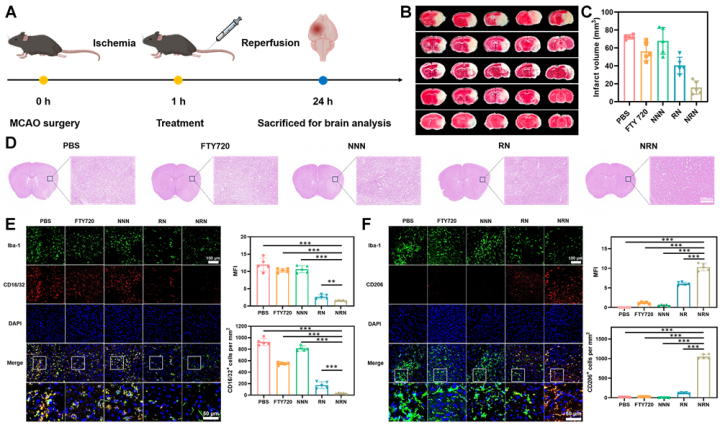
The research group designed and constructed a ROS-responsive polyfingolimod nanoprodrug coated with neutrophil membranes for targeted therapy of ischemic stroke reperfusion injury. On one hand, the neutrophil membrane-coated polyprodrug nanocarriers can effectively cross the blood-brain barrier due to their inherent inflammatory tropism, enhancing the drug delivery efficiency to the stroke site. On the other hand, the ROS responsiveness of the nanoparticles ensures selective drug release at the stroke site, thereby reducing the side effects of fingolimod after intravenous administration. The study showed that compared to free fingolimod, the polyfingolimod nanoprodrug significantly improves cognitive and motor abilities of stroke mice while exhibiting markedly reduced cardiac toxicity and infection risk. Single-cell RNA sequencing analysis revealed that the polyfingolimod nanoprodrug can achieve anti-inflammatory effects by regulating the expression of the key microglial gene Cebpb at the inflammation site. This research not only provides a new platform for the application of fingolimod in stroke therapy, but also offers new insights into the use of polyprodrug nanomedicines in disease treatment and diagnosis.
Paper Link: https://onlinelibrary.wiley.com/doi/10.1002/adma.202311803?af=R


