The team led by Professor Miao Yu from the School of Chemical Engineering and Technology and Professor Ye Sun from the School of Instruments has made significant progress in modulating the tumor immune microenvironment and advancing antitumor therapy. Their research, titled Long-Term Relapse-Free Survival Enabled by Integrating Targeted Antibacteria in Antitumor Treatment, has been published in Nature Communications. In this work, they successfully activated antitumor immune responses and restored antitumor immune surveillance through targeted intratumoral antibacteria combined with multimodal antitumor treatment, achieving long-lasting immunity and effective inhibition of distant tumors. This realization marks the complete cure of malignant tumors.
In recent years, the study of tumor-resident intracellular microbiota (TRIM) has emerged as a hotspot in understanding the initiation, progression, and recurrence of malignant tumors. TRIM can infiltrate tumors from surrounding tissues or the bloodstream, reducing the efficacy of chemotherapy drugs, exacerbating drug toxicity, and promoting the growth and metastasis of tumor cells. However, the impact of antibacteria within the tumor microenvironment (TME) on tumor inhibition remains unclear.
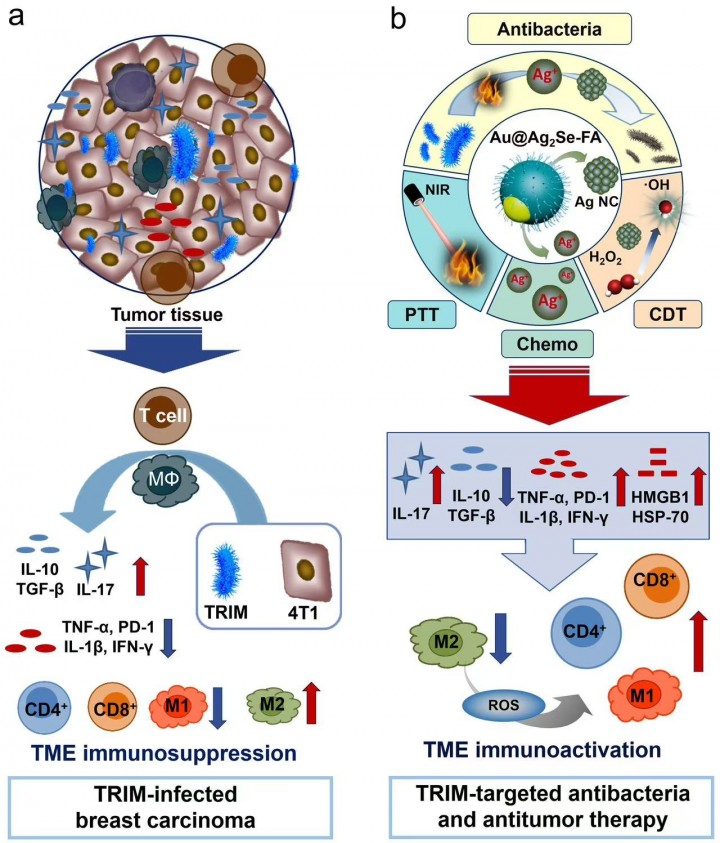
Professor Yu and Sun's team constructed models of two highly prevalent malignant tumors, breast carcinoma (4T1) and prostate carcinoma (RM-1), both infected with Escherichia coli (E. coli). They confirm that TRIM upregulates the expression of immunosuppressive cytokines [IL-10, TGF-β] and pro-inflammatory cytokines IL-17, while downregulates the expression of other pro-inflammatory cytokines [IL-12, TNF-α, and IFN-γ] and PD-1. It also reduces the number of T cells and M1-type macrophages in the tumor area while increasing the number of M2-type macrophages. In vivo experiments demonstrate that the presence of bacteria not only exacerbates immunosuppression in the tumor regions directly infected by E. coli but also significantly intensifies immunosuppression in the tumor regions of mice raised in non-sterile environments, leading to a substantial increase in tumor growth rates (Fig.1a).
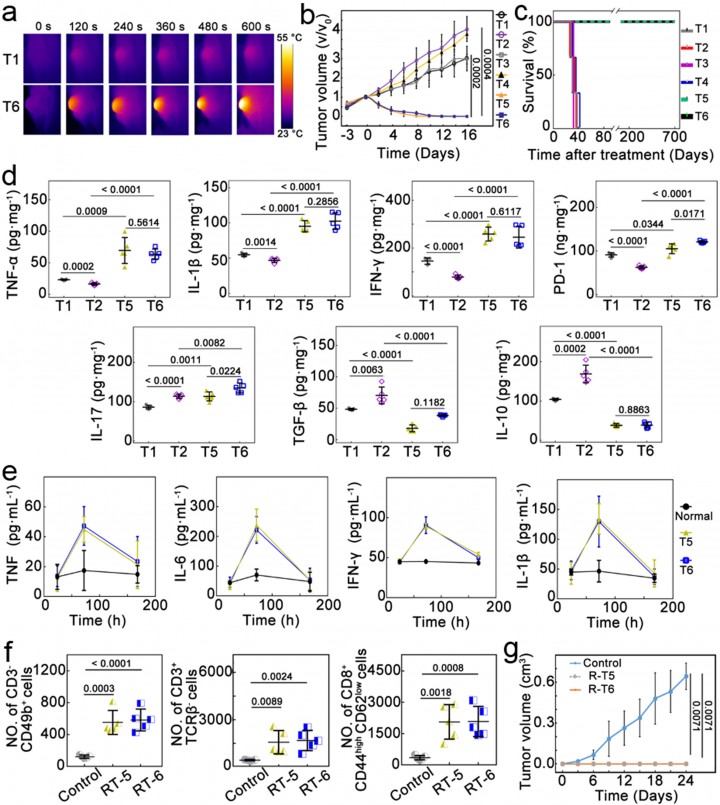
To address this challenge, the team designed and constructed a silver selenide shell-covered gold nanoagent and modified it with folic acid on its surface without the use of immune-stimulating drugs. This single agent can simultaneously achieve multimodal tumor suppression through photothermal therapy, chemodynamic therapy, and chemotherapy while enabling TRIM-targeted antibacteria (Fig.1b). By combining antibacterial and antitumor strategies, this approach effectively tackles the TRIM-induced issues of immunosuppression, excessive tumor growth, and recurrence. It successfully activates T cell immune responses and promotes the repolarization of M2-type to M1-type macrophages, triggering long-term antitumor immune memory. A relapse-free survival of >700 days is achieved (Fig.2). This work unravels the pivotal role of TRIM-targeted antibacteria in tumor inhibition and unlocks an unconventional route for immune regulation in the TME and a complete cure for cancer.
Paper Link: https://www.nature.com/articles/s41467-024-48662-x


