HIT All media (by courtesy of HIT Center for Life Sciences): Recently, Professor Chen Zheng and his research team from HIT Center for Life Sciences achieved a discovery which reveals the mechanism by which METTL3 affects the progression of NASH. The results of this study were published in Nature Communications in an report entitled The methyltransferase METTL3 negatively regulates nonalcoholic steatohepatitis (NASH) progression.
About 25% of patients with NAFL develop NASH and it can even develop to cirrhosis and hepatocellular carcinoma. Little is known, however, about which genes regulate the NAFL-to-NASH transition. Chen Zheng's group found that RNA methyltransferase METTL3 plays an important role in the NAFL-to-NASH transition. Liver-specific Mettl3 knockout mice developed a NASH phenotype under HFD feeding which is characterized by higher serum ALT activities, more pronounced fatty liver, liver injury, inflammation, and liver fibrosis. However, compared with Mettl3flox/flox mice, it didn’t show NASH which indicates that Mettl3-HKO mice are more sensitive to MCD-induced NASH and displayed more severe NASH.
Hepatic deletion of Mettl3 accelerates free fatty-acid uptake by increasing CD36 expression and leads to hepatic lipid accumulation. Hepatic deletion of Mettl3 enhances CCL2 expression which suggests that hepatic deletion of Mettl3 accelerates liver injury and inflammation. Inhibition of CD36 and CCL2 ameliorates NASH progression in Mettl3-HKO mice. Hepatic overexpression of Mettl3 ameliorates MCD-induced NASH by inhibiting the expression of CD36 and CCL2.
RNA-seq, ATAC-seq and m6ARIP-seq data show that METTL3 can regulate the transcription of Cd36 and Ccl2 in the liver independent of its methyltransferase enzyme activity. METTL3 interacts with HDAC1/2 and regulates H3K9ac and H3K27ac levels in the promoters of Cd36 and Ccl2. TSA reverses the suppression of Cd36 and Ccl2 expression mediated by METTL3 overexpression.
These data indicate that TNFα/CDK9-mediated phosphorylation of METTL3 may contribute to the reduction of nuclear METTL3 levels, decreased the interaction between METTL3 and HDAC1/2, in NASH livers. This study also suggests that METTL3 is a negative regulator of NASH pathogenesis and may serve as a drug target for the treatment of NASH.
Xinzhi Li, postdoctoral fellow at HIT Center for Life Sciences, and Bingchuan Yuan, HIT doctoral candidate, are the co-first authors of this paper, and Zheng Chen, research fellow , is the corresponding author of this paper. Min Lu, Senior Engineer of Experimental Teaching Center, College of Life Science and Technology, and Yuqin Wang, Na Ding, Chunhong Liu and Ming Gao, graduate students of Zheng Chen's group, participated in this research. This project was also supported by Dr. Zhicheng Yao from The Third Affiliated Hospital of Sun Yat-sen University, postgraduate students Shiyan Zhang and Yujun Zhao from the Shanghai Institute of Materia Medica, Chinese Academy of Sciences, and Liwei Xie, research fellow from the Guangdong Institute of Microbiology. The research is funded by the National Science Foundation of China and HIT research funds.
The original link:https://www.nature.com/articles/s41467-021-27539-3
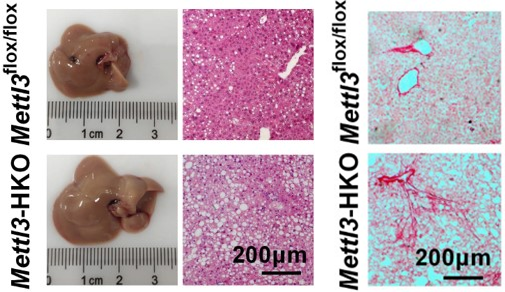
Mettl3-HKO mice develop more severe fatty liver and liver fibrosis under HFD
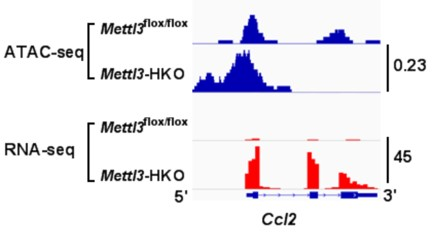
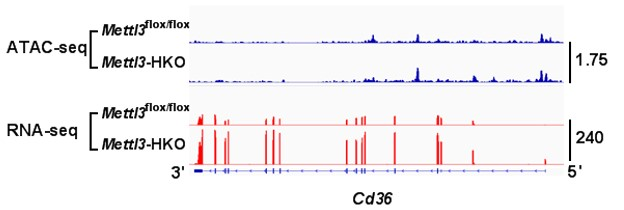
ATAC-seq and RNA-seq showed that the deletion of Mettl3 promotes Cd36 and Ccl2 transcription. RNA-seq data also showed that both Cd36 and Ccl2 gene transcription




