A research team led by Professor Xiaojun Han from the School of Chemistry and Chemical Engineering at Harbin Institute of Technology (HIT) and the State Key Laboratory of Urban Water Resources and Environment, has achieved a major breakthrough in artificial cell research by successfully constructing a functional glycolytic metabolic pathway within synthetic cells. Published in the Journal of the American Chemical Society (JACS) under the title Construction of Glycolysis Metabolic Pathway Inside an Artificial Cell for the Synthesis of Amino Acid and Its Reversible Deformation, this work addresses a longstanding challenge in the field and provides foundational insights for developing artificial cells with complex metabolic functions.
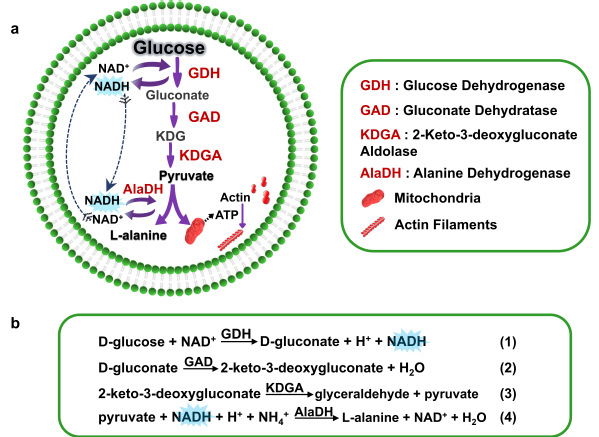
Glycolysis, a critical energy-harvesting pathway essential for life, has been notoriously difficult to replicate in artificial cell systems. Building a functional pathway from glucose to pyruvate within artificial cells has remained a major hurdle.
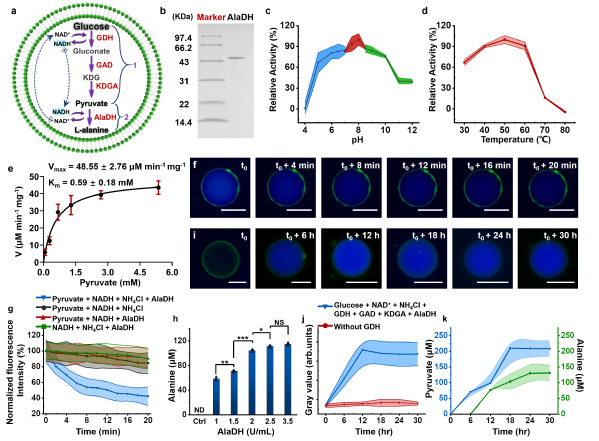
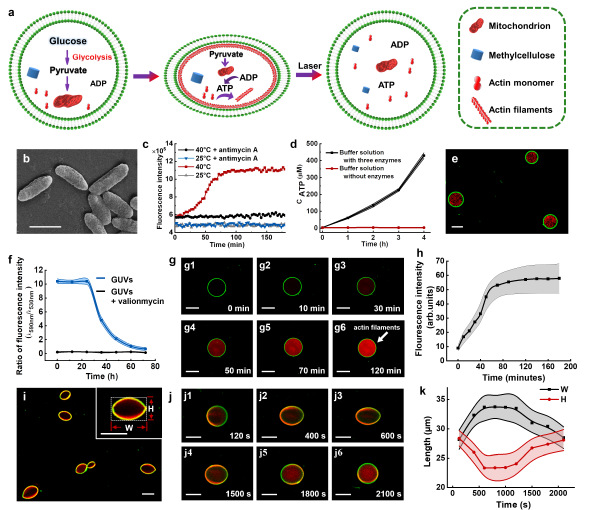
To overcome this, Prof. Han’s team drew inspiration from archaeal glycolysis pathways. By encapsulating key enzymes within artificial cells, they successfully achieved glucose-to-pyruvate conversion. Leveraging pyruvate as a metabolic hub, the team further synthesized alanine—a physiologically vital amino acid—by integrating alanine dehydrogenase into the system. Remarkably, the researchers combined glycolysis pathways, mitochondria, and actin proteins within the artificial cells. Glycolysis-generated pyruvate stimulated ATP production in the mitochondrial, which in turn drove actin monomers polymerization into filaments. This process induced a reversible shape change: spherical artificial cells transformed into ellipsoids under ATP activation and reverted to their original form upon laser-induced actin filament depolymerization.
This breakthrough not only demonstrates the first functional integration of glycolysis in artificial cells but also establishes a framework for designing synthetic cells with dynamic, life-like behaviors. The study opens new avenues for creating autonomous artificial cells capable of complex metabolic tasks, advancing synthetic biology and biomedical applications.
Paper Link: https://pubs.acs.org/doi/epdf/10.1021/jacs.4c06227