A collaborative study led by Professor Yuanzheng He’s team from the Life Science Center of Harbin Institute of Technology (HIT) and Professor Huaqiang Xu’s group at the Shanghai Institute of Materia Medica, Chinese Academy of Sciences, has revealed the structural basis of ligand recognition and G-protein coupling for the lysophosphatidylinositol (LPI) receptor GPR55. The findings, published in the prestigious journal Cell Research under the title “Structural insight into GPR55 ligand recognition and G-protein coupling”, provide critical insights into GPR55 signaling mechanisms, paving the way for novel therapeutics targeting neurological and metabolic disorders.
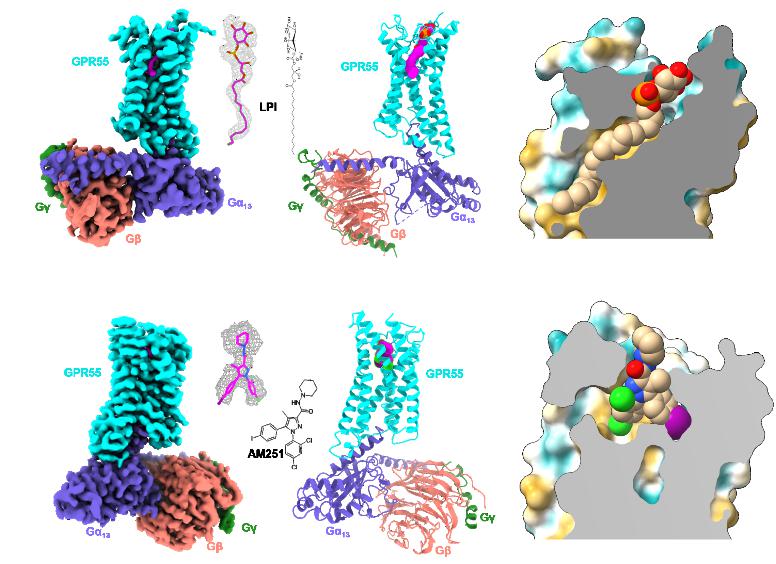
GPR55 plays pivotal role in both the nervous and metabolic systems. In the nervous system, it regulates sensory perception, cognition, and pain response, and is a therapeutic target for Parkinson’s disease. In metabolic processes, it governs insulin secretion, gut inflammation, and blood sugar levels, making it a key focus for treating diabetes and non-alcoholic steatohepatitis. Initially debated as a novel cannabinoid receptor, GPR55 is now recognized to bind LPI as its endogenous ligand.
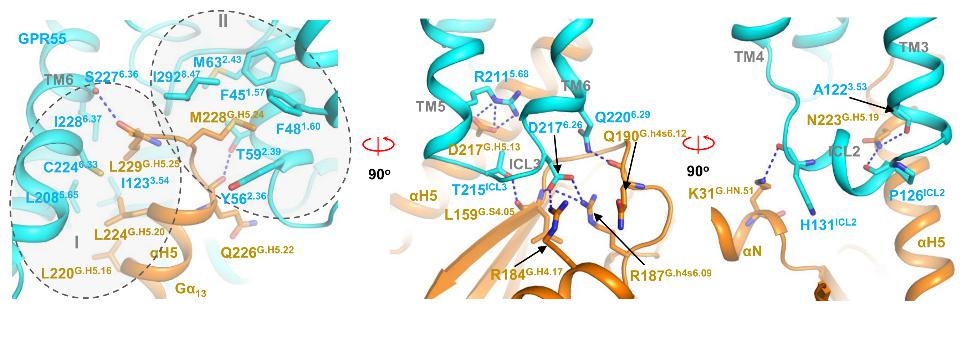
Using single-particle cryo-electron microscopy (cryo-EM), the team resolved the structures of GPR55 bound to its native ligand LPI and a synthetic ligand AM251 in complex with the G13 protein at resolutions of 2.85 Å and 3.19 Å, respectively. The study revealed that LPI forms an extensive polar interaction network with key amino acids in the receptor’s binding pocket, while AM251 binds more loosely via hydrophobic interactions in the mid-region, explaining its weaker activity compared to LPI. Strikingly, structural comparisons confirmed that GPR55’s ligand-binding mode is distinct from classical cannabinoid receptors, conclusively resolving the long-standing debate regarding its classification. Further analysis identified a unique “methionine pocket” critical for GPR55’s coupling to the Gα13 protein.
“This work not only clarifies the molecular basis of GPR55 signaling but also opens new avenues for designing drugs targeting neurological and metabolic diseases,” said Professor Yuanzheng He. The findings could accelerate the development of precision therapies for conditions like Parkinson’s, diabetes, and inflammatory disorders.
The research marks a milestone in understanding GPR55’s dual roles and underscores HIT’s leadership in bridging structural biology and translational medicine.
Paper link: https://www.nature.com/articles/s41422-024-01044-w